Quality Management made simple for your business
A risk-based QMS offering industry best practices to manage your compliance needs and meet current regulatory requirements.
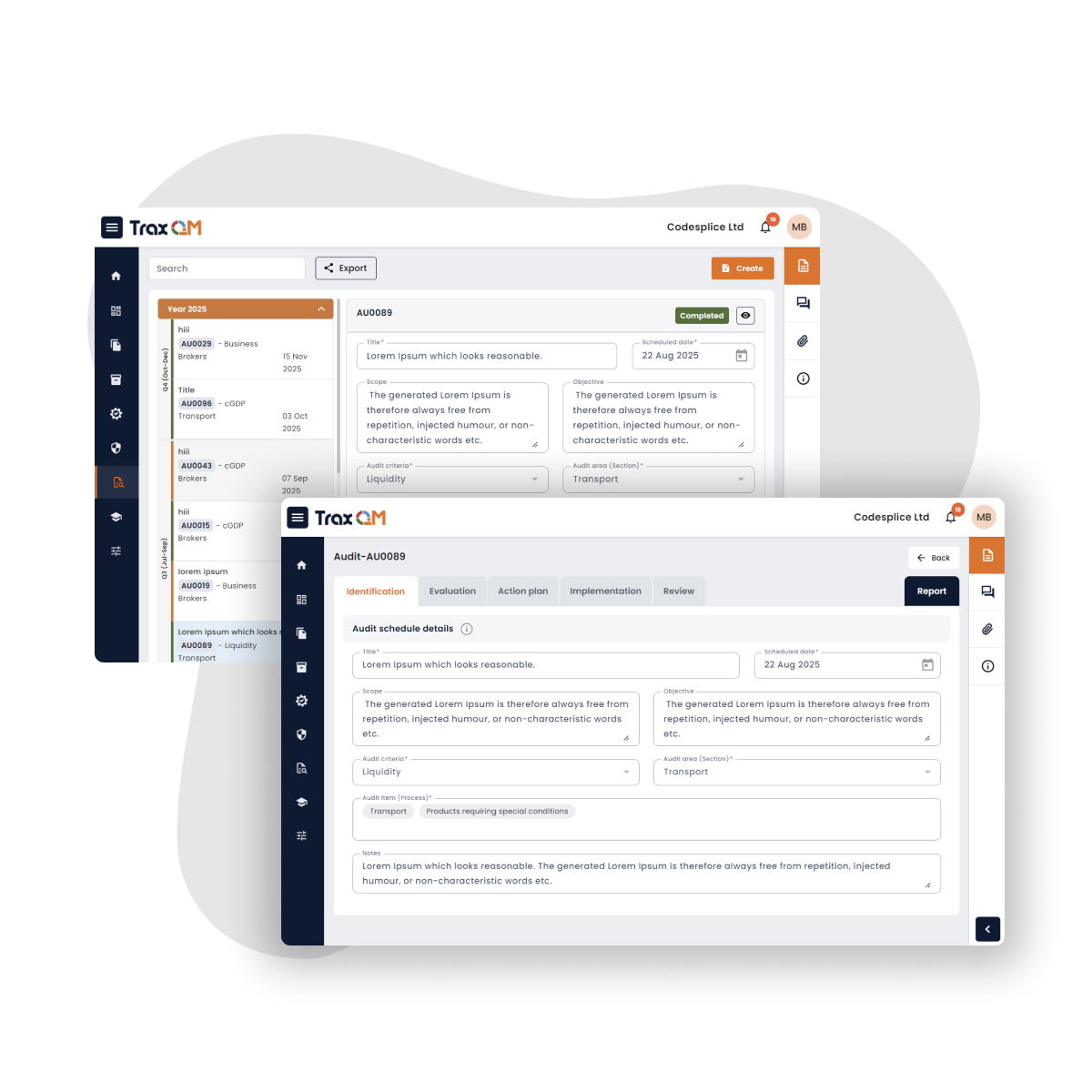
Everything you need to run compliant processes.
TraxQM provides a platform for companies to take a proactive approach to regulatory compliance and risk management.
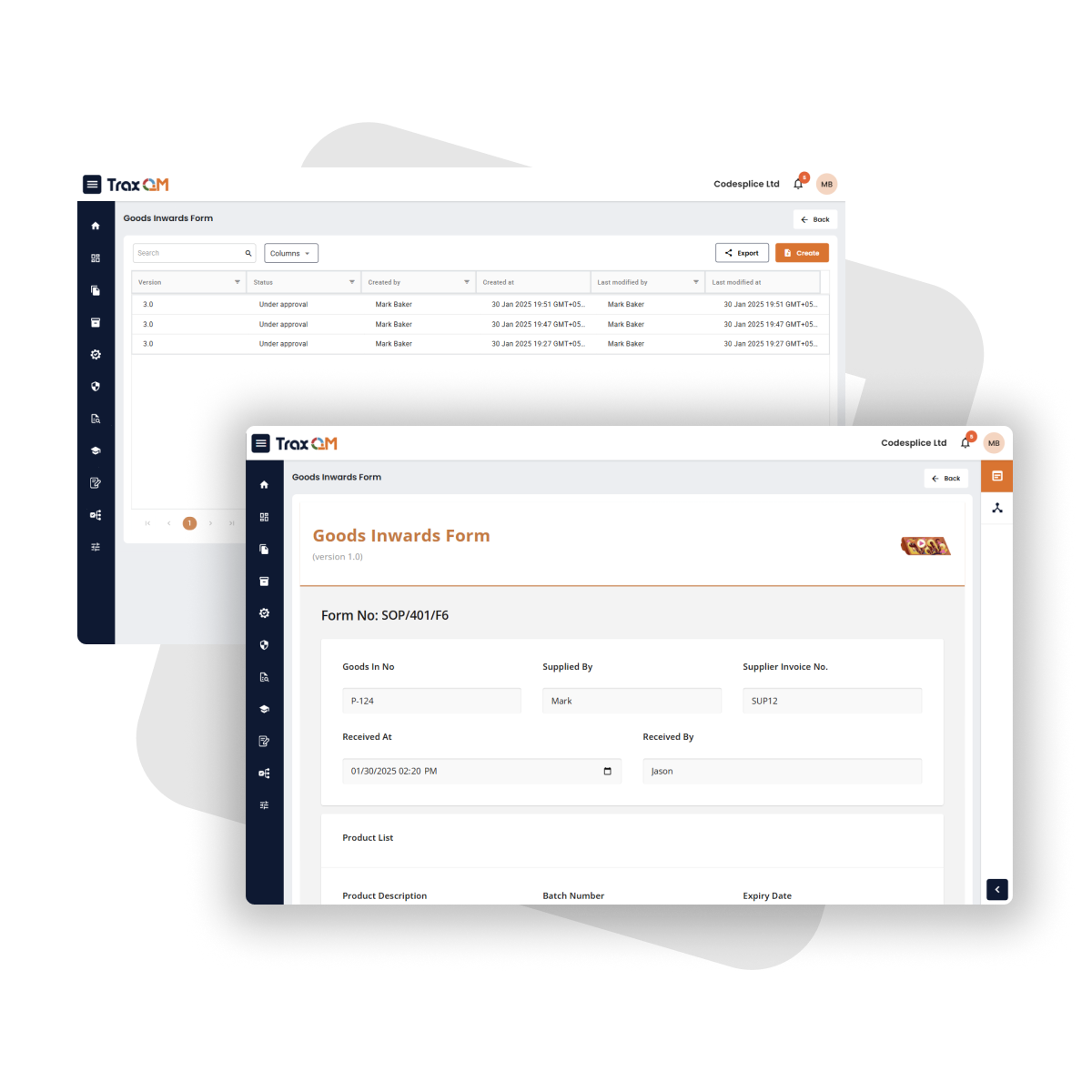
Full support for MS Office files. Data integrity supporting ALCOA principals ensured with automated version control, 21 CFR Part 11 enabled signatures and an audit trail
AI-Powered Features of TraxQM
TraxQM leverages AI to enhance risk management, compliance and decision-making ensuring proactive quality control and regulatory adherence.
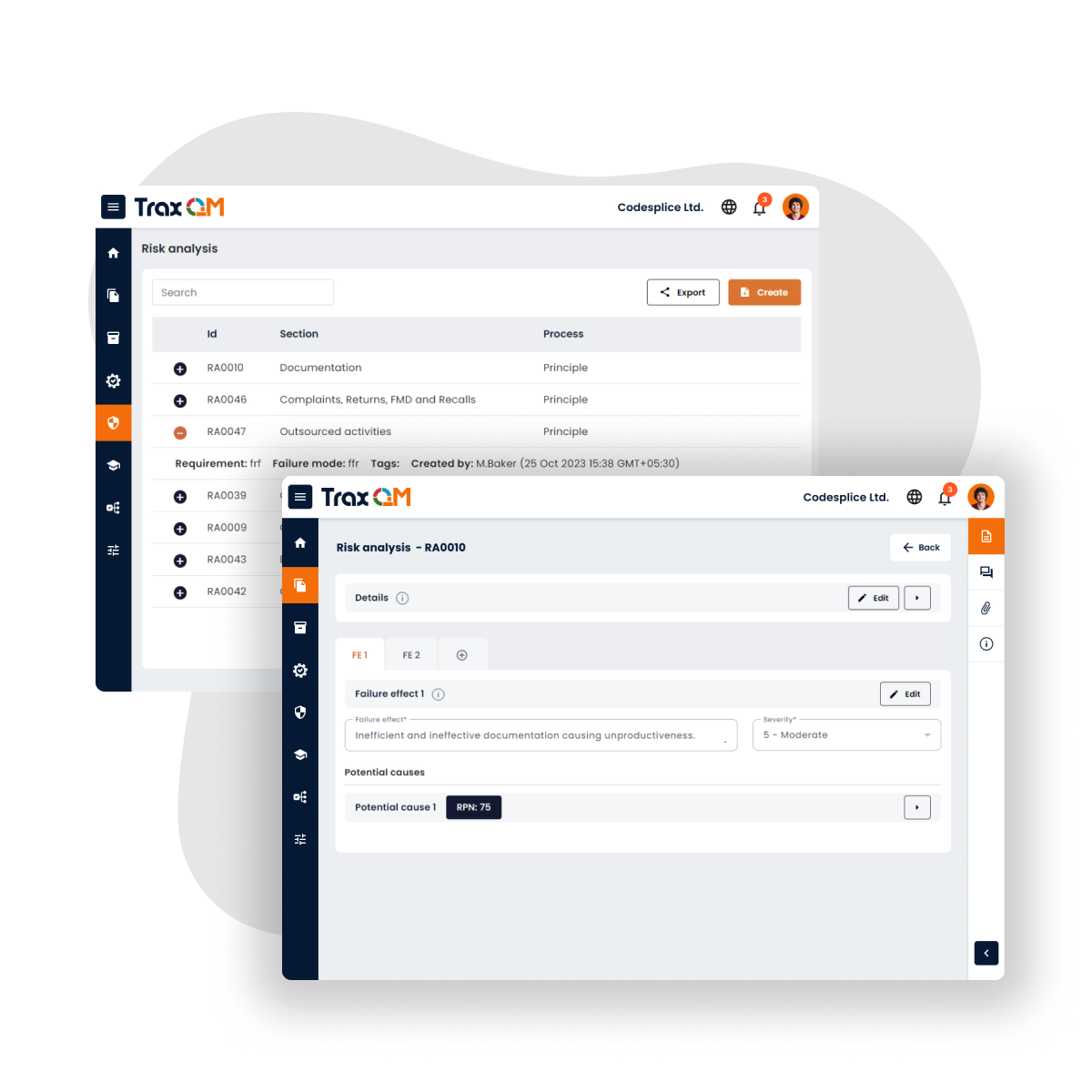
Risk Management
AI-driven risk analysis for proactive quality control
AI analyses existing risks and suggests targeted control measures to mitigate potential issues. This helps our clients to enhance compliance, strengthen quality processes, and proactively address risks before they lead to non-conformance.
KPIs
Intelligent KPI recommendations
AI suggests relevant Key Performance Indicators (KPIs) based on organisational objectives and risk profiles. This ensures a focused approach to monitoring, optimising resources and improving critical areas of business success.
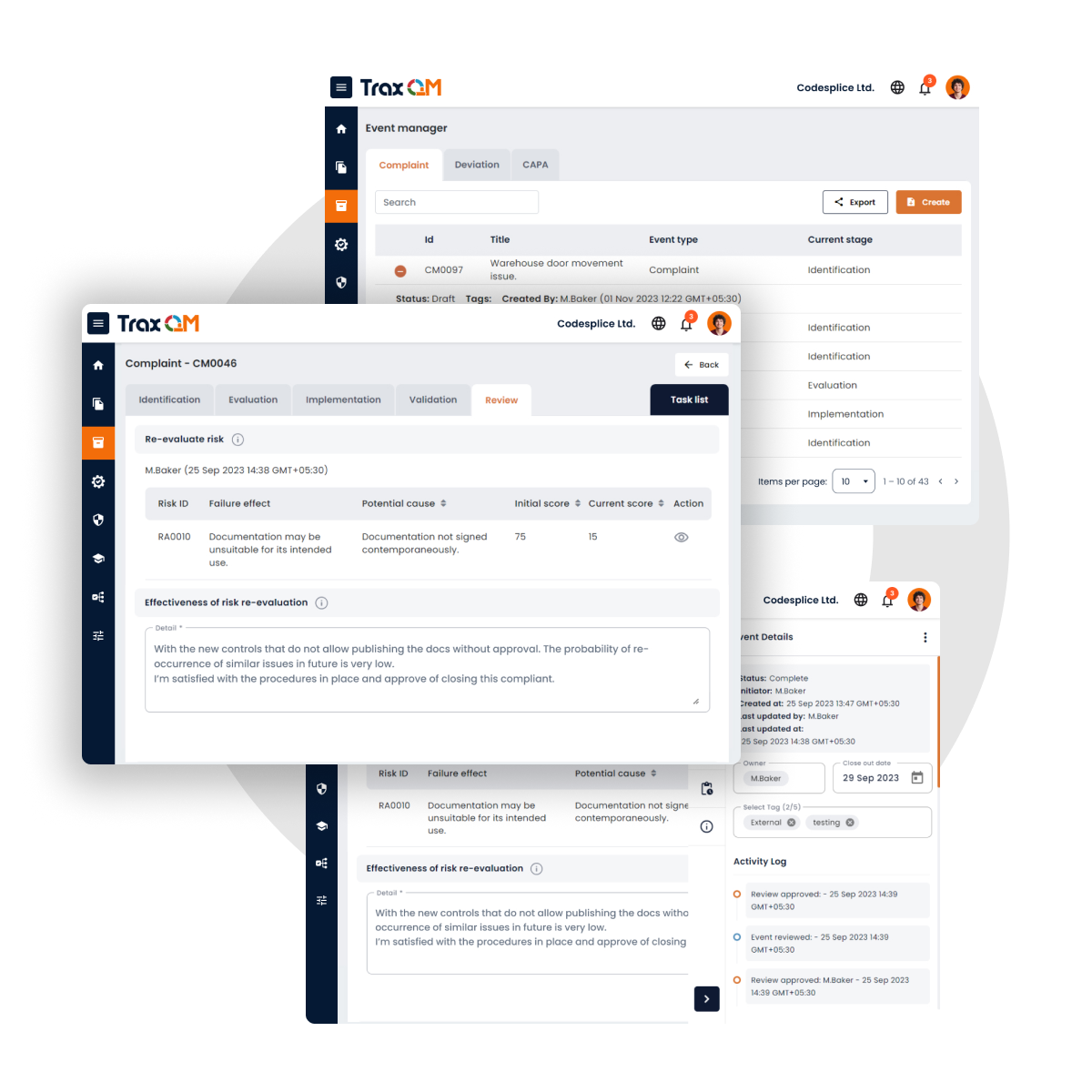
Document Management
AI-powered document review for GxP compliance
AI-driven system enables users to review documents and receive feedback on content quality and compliance with GxP guidelines. This ensures that documents meet regulatory standards, improving accuracy and adherence to industry best practices.
Training
Generate comprehensive training with AI
Quality content in just a few clicks? Check! Text, images, tests—cover every element of course creation and accelerate along the path to L&D success. Take pride in offering complete courses right on time.
AI-driven risk analysis for proactive quality control
AI analyses existing risks and suggests targeted control measures to mitigate potential issues. This helps our clients to enhance compliance, strengthen quality processes, and proactively address risks before they lead to non-conformance.
Intelligent KPI recommendations
AI suggests relevant Key Performance Indicators (KPIs) based on organisational objectives and risk profiles. This ensures a focused approach to monitoring, optimising resources and improving critical areas of business success.
AI-powered document review for GxP compliance
AI-driven system enables users to review documents and receive feedback on content quality and compliance with GxP guidelines. This ensures that documents meet regulatory standards, improving accuracy and adherence to industry best practices.
Generate comprehensive training with AI
Quality content in just a few clicks? Check! Text, images, tests—cover every element of course creation and accelerate along the path to L&D success. Take pride in offering complete courses right on time.
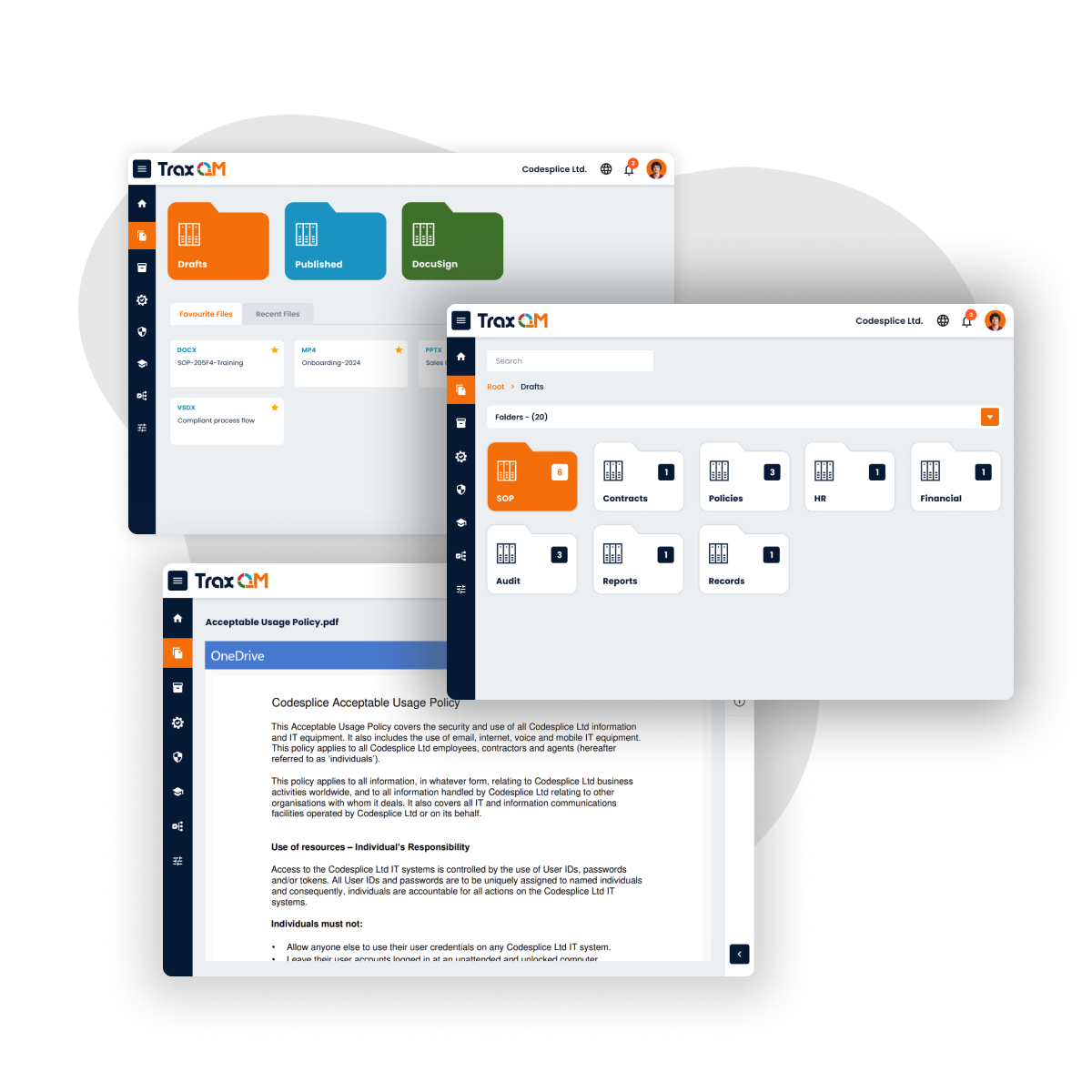
Provides powerful data visualisation tools that offer a comprehensive overview of your organisation's quality health. Empower your management team with actionable insights and real-time data to drive informed decision-making and maintain regulatory compliance.
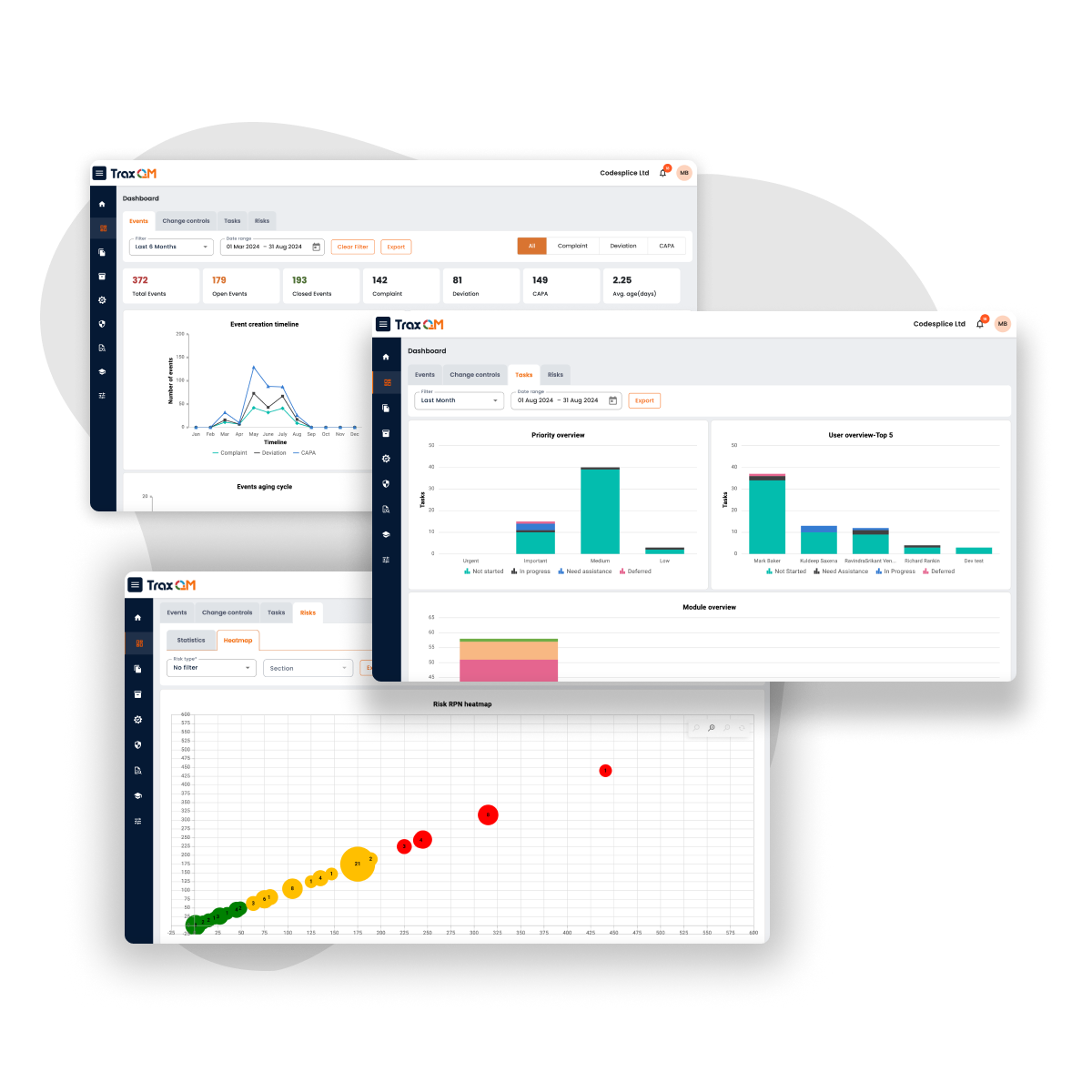
Keep the whole team informed
TraxQM offers teams the ability to meet quality requirements in a formal manner via builtin training module, task based management and 21-CFR enabled integrated DocuSign support.
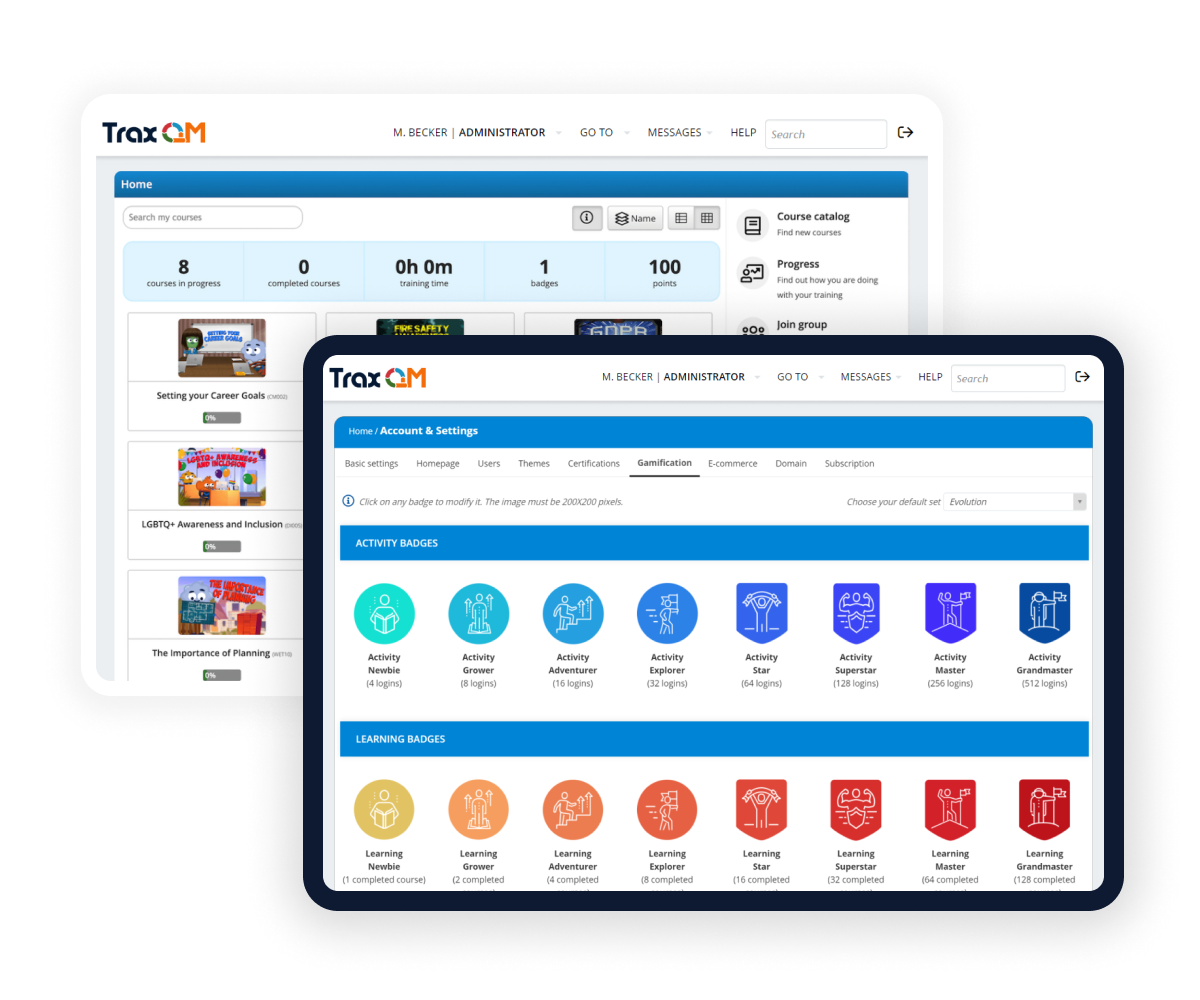
Training
Create, manage and measure all of your training in one seamless platform.
Create from a collection of over 600 ready-made courses or upload your existing materials. Access reports about staff training and automatically assign courses.
Tasks
Make it easy for your team to focus on tasks via digital Kanban boards.
Manage actionable and targeted work instructions using tasks. Tasks allows the user to specify assignees, priority, time frames and add comments and attachments.

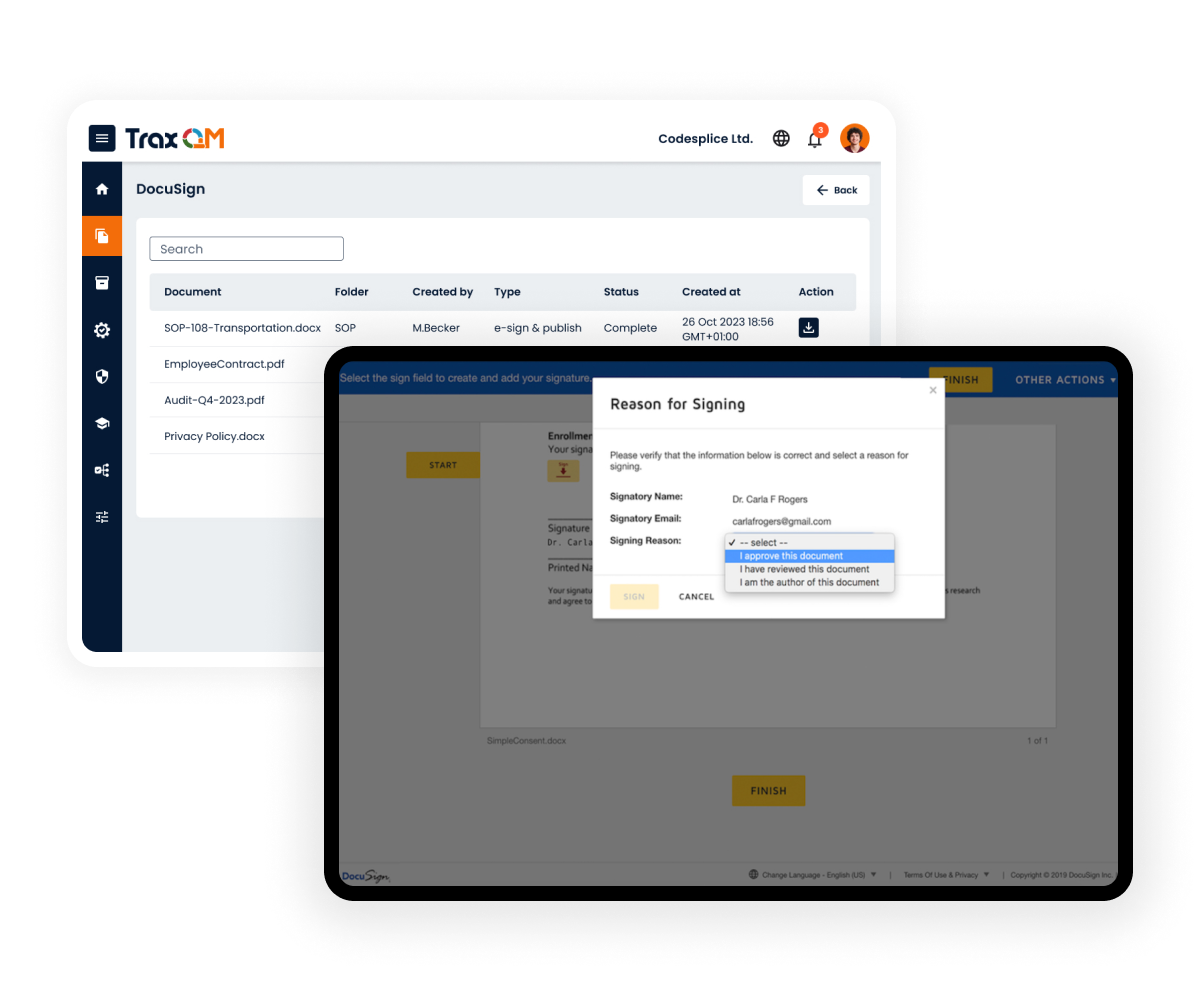
DocuSign
The eSignature solution trusted by hundreds of millions of users
The electronic signatures with FDA 21 CFR Part 11 Compliance allows users to use them for document approvals or to legally digitise external agreements.
Create, manage and measure all of your training in one seamless platform.
Create from a collection of over 600 ready-made courses or upload your existing materials. Access reports about staff training and automatically assign courses.
Make it easy for your team to focus on tasks via digital Kanban boards.
Manage actionable and targeted work instructions using tasks. Tasks allows the user to specify assignees, priority, time frames and add comments and attachments.
The eSignature solution trusted by hundreds of millions of users
The electronic signatures with FDA 21 CFR Part 11 Compliance allows users to use them for document approvals or to legally digitise external agreements.

GDP Pharma Consulting are pleased to recommend TraxQM as a trusted partner to provide a robust and practical risk-based e-QMS for WDA holders. The platform offers the tools required to effectively operate and maintain a GDP compliant QMS, fully meets ALCOA requirements and can support successful regulatory inspections.
--- Peter Coombs and Claire Glenisterex-MHRA Inspectors and founders of GDP Pharma Consulting

Frequently asked questions
If you can’t find what you’re looking for, contact our support team.
Why should I choose TraxQM?
To manage cost and complexity of compliance with a SaaS application. Digitise your paper-based workflows using a structured approach to data entry. Use dash boards and charts to view key data.
Is TraxQM Risk based?
Absolutely, Risk-based quality management is integral to TraxQM. Record objectives and measurable controls to manage deviations. Weighed severity, occurrence, and detection scores will indicate level of risk.
Can I manage compliance deficiencies?
Aligned to regulatory expectations, deficiencies can be managed in a measured way in areas such as risk assessment, formal definition of severity, detail within controls and methodologies for RCA.
Are documents and records secure?
Published documents with or without electronic signatures are automatically version controlled through a review and approve stage, meeting ALCOA requirements.
Who can use TraxQM?
Registered users of a quality driven company can view, edit, review, or approve documents and events. Comments board and tasks allow for companywide management of quality objectives.
Can TraxQM be used for multiple companies?
Yes, the application can be used for identifying files, folders, and events in multi company, multi-site environments.
Do we need a DocuSign account?
Users can continue to use their existing DocuSign accounts. In case they are new to DocuSign, they will need to create a free tier account. TraxQM includes the 21-CFR compliance feature.
What happens to my current documents and records?
Existing documents can be uploaded to the document repository and published with a version controlled.
Does TraxQM support Regulatory Inspections?
The design, guided by former MHRA inspectors, industry best practices, domain experience, regulatory guides, ICH standards and white papers reduces ambiguity and supports inspections.
Is the product validated/qualified?
Fully tested and qualified for design, installation, and performance, the documentation will include a URS, user guide and validation summery report. These will support user validations/qualifications.
How do I train to use TraxQM?
Onboarding webinars and inductions will be arranged. Training is also provided through a user guide and videos in the training module.
How long are my documents stored for?
Documents can be archived at any time and can only be deleted after a five year period. Deletion of documents is optional and has no time limit.